Color
![[url=https://pixabay.com/en/yellowstone-national-park-wyoming-1589613/]"Yellowstone"[/url] by 12019 is in the [url=http://creativecommons.org/publicdomain/zero/1.0/]Public Domain, CC0[/url]
Yellowstone national park. Many topics from this chapter can be seen in this photo, and it is beautiful to look at too!](https://www.geogebra.org/resource/Xg5beZEB/Bf4f4kDsYtoKy18r/material-Xg5beZEB.png)
What can we see?
Physicists often refer to all electromagnetic radiation as light. Some people are only willing to use the term "light" to describe light that is visible. I will use the term to mean both visible and invisible. One reason for this is that there is no difference between light we can see versus light we can't see except that the wavelength is different.
Human eyes without any visual defects can detect light in the range from 400 nm to 700 nm in wavelength. While there is in principle no limit to the range of wavelengths of light found in nature, we can find around 26 orders of magnitude of variation without searching too hard. If we were to construct a piano keyboard that would produce light rather than sound when we strike a key, there would be around 87 octaves on the piano such that it could "play" the range of light found in nature. A real piano has 7 octaves on its keyboard. Of the 87 octaves on our light-producing piano, we can only see less than a single one of them with our eyes.
What is Color?
I will start by saying that color is not found in nature. That may seem odd to you. Yet the fact remains that color is an imagination or creation of the human mind. It only exists in your mind's eye. Nature is full of oscillating fields - electromagnetic ones are light, of course. Light causes atoms and molecules to oscillate when they meet. If the oscillation frequency of light matches a resonance in an atom or molecule, the light will get absorbed into the system. Absorption means a change in shape for biological molecules. A change in shape is called a conformational change. In the case of sensory molecules in your eyes, there are three types of receptors that are capable of absorbing light in different ranges of frequencies. If absorption takes place in any of those molecules, conformational changes take place, and subsequently electrical signals are sent to the brain, and your brain gets the news that energy was absorbed. That is vision. What you "see" is the picture your mind creates of that incoming data.
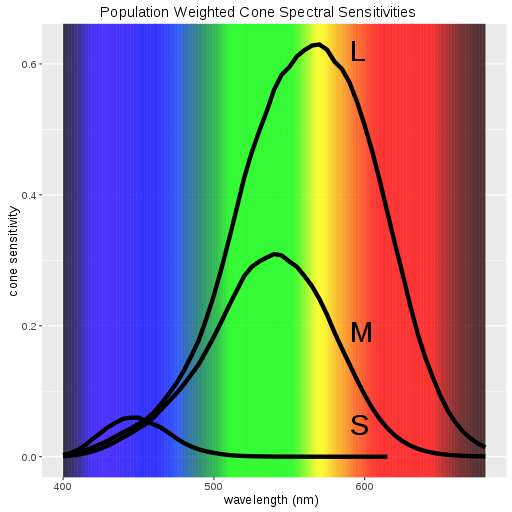
The three different molecules on the retina have peak sensitivities (or ability to absorb light) for red light, green light and blue light respectively. This is exactly the reason why we use RGB codes to describe color in photographs and on computer monitors.
So what is color? It is just the way your brain decides to interpret different combinations of relative activation of the three different types of light receptors on your retinal surfaces. Personally, I like it that the brain represents this activation as color rather than a bar graph or by displaying a wavelength in nanometers or something!
One related fact to the absorption curves seen below is that green/yellow light appears brightest to our eyes among all colors. Since we aren't as sensitive to the blue or red ends of the spectrum, light of those colors would have to carry much more energy than green light in order for it to appear as bright.
Looking at the plot below, for instance, it is apparent that yellow/green light is strongly absorbed by both the M and L receptors such that the combined sensitivity is around 0.9 - which comes from roughly 0.6+0.3. Compared with blue light sensitivity where the peak is at maybe 0.06, this means green light will appear around 15 times brighter to us even when it contains the same energy. Red falls somewhere between blue and green in this regard.
Retinal Absorption
Why is the Sky Blue?
The sky is blue due to something called Rayleigh (pronounced Ray-Lee) scattering. If you investigate the details of Rayleigh scattering, it sound illegal according to the laws of nature. What I mean by that is that light manages to interact with air molecules in a way in which the light leaves the molecules unchanged (in the same quantum state). But that sounds like no interaction at all. Visible light does not have enough energy to actually excite the electrons inside the diatomic molecules that predominantly make up our atmosphere.
In spite of this, what happens is that there is borrowed energy that can be taken out on loan from nature in order to allow the excitation to occur anyway - but only for a very brief period of time. This is called a virtual state of excitation since it is only allowed to exist for a very short time governed by a principle called Heisenberg's uncertainty principle that we'll discuss again later in the course. That very same principle makes it impossible to actually measure such excited states, which is why the term "virtual" is used, since it implies that it is not a physically observable state and doesn't seem as real as other measurable states. Yet the interaction takes time and has a consequence, which is the scattering or deflection of the path of the light in the atmosphere.
According to Heisenberg's uncertainty principle , the less energy that needs to be borrowed the longer the virtual excitation can last and the stronger the scattering interaction will be. On the right side of the equation is what's called the reduced Planck's constant. Since blue light is more energetic than red light, it needs to borrow less energy to create these virtual excited states of air molecules than red light does, so blue interacts or scatters more strongly than red light.
The details of Rayleigh scattering are complex, but what is easy to remember is that the interaction depends on the frequency to the power. Since violet light is just under twice the frequency of red light, it means we have around (it would be if it was truly double) more scattering of blue than red light in air.
So back to the blue part. The effect is easiest to understand if we just think in absolutes and suppose that blue light always scatters (or gets bounced around) and red light never does. If you think in absolutes like this, then it's clear that red light could only ever reach you if it comes right at you from the sun. On the other hand blue light would bounce all around the atmosphere and everywhere you look you'd see some blue light coming your way like a whole bunch of pinballs bouncing around hitting you from every direction. Reality isn't absolute like that, but relatively speaking there is much more blue than any other color coming from all directions, which makes the sky that color.
Why are Sunrises and Sunsets Red?
The flip side to blue being scattered strongly and red light passing rather unencumbered through the atmosphere is that when we look rather directly at the sun we should expect to see not the sun's true color, but rather its true color minus some blue. The more atmosphere we have to look through to see the sun, the more blue should be subtracted out.
When the sun is rather directly overhead, the layer of atmosphere between you and the sun is rather shallow. The density of the atmosphere drops exponentially with altitude. So even though you might read somewhere that the atmosphere is roughly 10s of kilometers thick, most of the air is near the ground in the first 10 km.
When you look at the sun when it's near the horizon you are looking though 100s of kilometers of thick, surface atmosphere in addition to the outer layers. That gives the atmosphere plenty of opportunity to scatter away the blue component of the spectrum before the sunlight reaches your eyes. That leaves the red end of the spectrum, which gives sunrises and sunsets their red color.
Why is Water Aquamarine Colored?
The color of water depends on the fact that water is actually opaque (not transparent) to most wavelengths of electromagnetic radiation (light). It turns out that the visible spectrum is a very narrow band in which water looks relatively transparent. I say relatively because it does absorb visible light, and that absorption is strongest on the red end of the spectrum. It is smallest in the yellow-green wavelengths and then absorbs more in the blue-violet, but less so than red.
A plot of water's absorption versus wavelength is seen below. Take note that the axes are logarithmic. That means that each major division is 10x more than the one before it! Also note the deep dive in absorption right at the visible wavelengths. Low absorption means transparency. The way to understand the units on the y-axis is that is tells us about the probability of absorption. How this works is the following: At the peak value of the corresponding fraction light T that manages to penetrate or transmit through a depth of water is given by , where is the absorption coefficient from the graph. So when the absorption coefficient is large, light doesn't go very far through water before being absorbed. When the coefficient is small (as in the yellow-green region) it can go quite far before being mostly absorbed. The largest T can get is unity. That just means all the light made it through. T=0.5 would mean half the light intensity makes it through.
![[url=https://commons.wikimedia.org/w/index.php?search=water+absorption&title=Special%3ASearch&profile=default&fulltext=1#/media/File:Absorption_spectrum_of_liquid_water.png]"Water Absorption Spectrum"[/url] by Kebes is licensed under [url=http://creativecommons.org/licenses/by-sa/3.0]CC BY-SA 3.0[/url]](https://www.geogebra.org/resource/vdVkDpQF/tqLPRXpMoHQt17XJ/material-vdVkDpQF.png)
Why are Clouds White?
Clouds are white because in spite of water having a tendency to slightly absorb the red end of the spectrum as we discussed above, the light has to pass through a rather deep section of water for that to become noticeable. That's why a glass of water doesn't appear colored, and if you think it does, it's either dirty or it's the glass that is colored. So since clouds are water droplets and since they are essentially transparent, the only effect they can have on light is to deflect its path. The light can bounce off the spherical droplets in a way that looks like diffuse scattering. Light may also pass through the droplets slightly deflected or refracted as we call it. It can also do a combination of these things which as we will discuss later in the book will lead to rainbows in special cases. For now you can think of clouds as a means of taking all wavelengths of light and scattering the light in every which direction uniformly. This gives clouds a white color.
When storm clouds become dark blue/gray, the depth of water within them is enough that absorption starts becoming noticeable. The light bounces around so much inside the clouds (and heats them up) that very little is reflected back to us. This gives them that dark, ominous appearance.
As you can see in the picture of Yellowstone national park at the beginning of the chapter, there are lots of factors leading to the color of clouds - including reflection and partial transmission of blue sky, reflection of direct sunlight, reflection of light coming from earth's surface features, etc. It all makes for a beautiful show during sunrises and sunsets - no two of which will ever be the same.